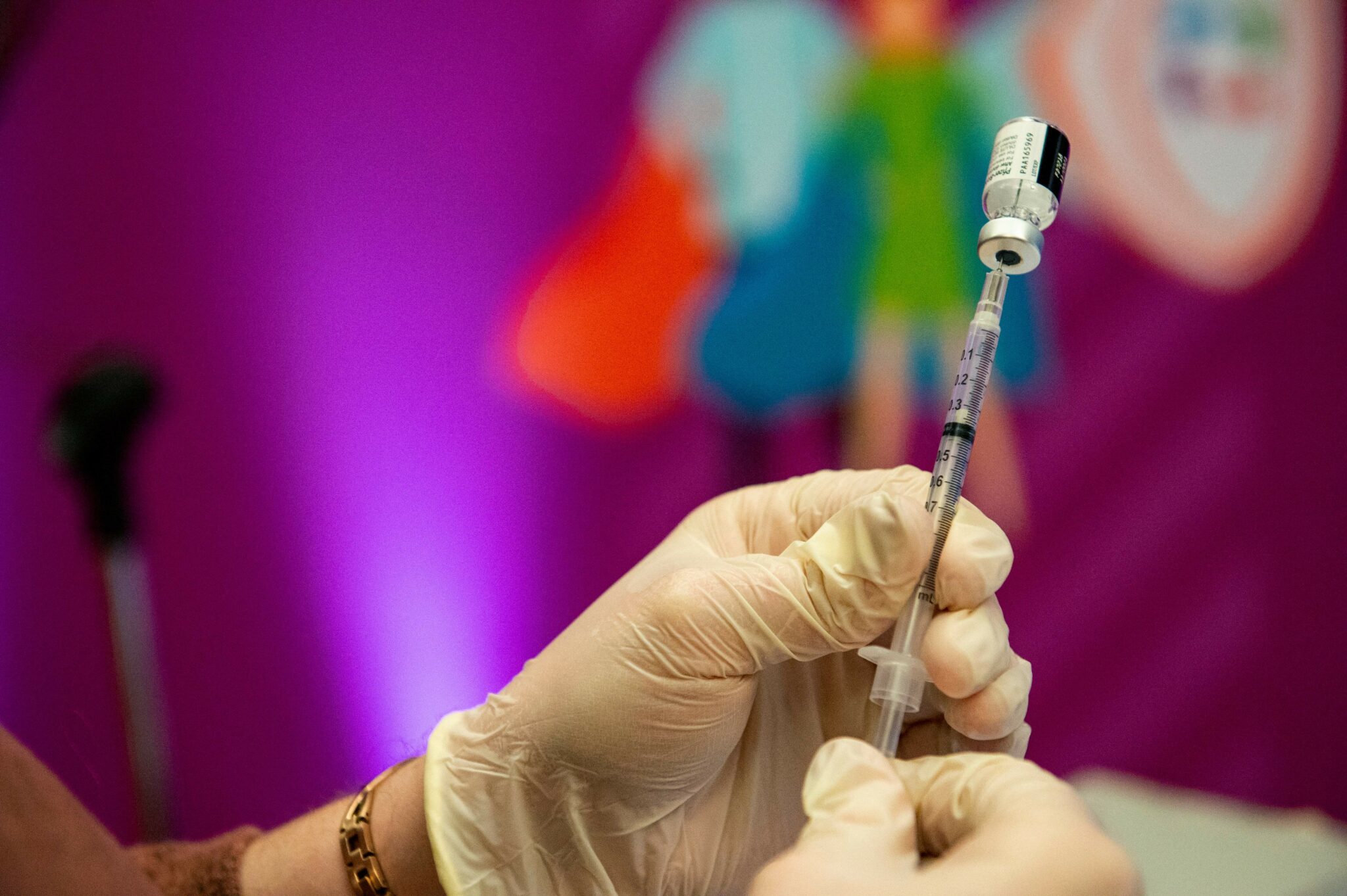
- Pharmaceutical giant Pfizer is seeking FDA approval for a first-of-its-kind vaccine aimed at protecting infants from the respiratory syncytial virus (RSV), but trial data shows the shot failed to meet one of its two main goals.
- The Bivalent Prefusion F Vaccine developed by Pfizer is administered to expectant mothers during the second half of their pregnancy to protect infants against RSV.
- According to the late-stage trial data, the experimental vaccine was 82% effective in preventing severe cases of the potentially deadly virus in infants 90 days after birth, but that dropped to 69% efficacy in preventing severe infections up to 180 days after a baby is born.
Trending
Pfizer Seeks FDA Approval Of First Vaccine To Protect Infants By Injecting Pregnant Mothers